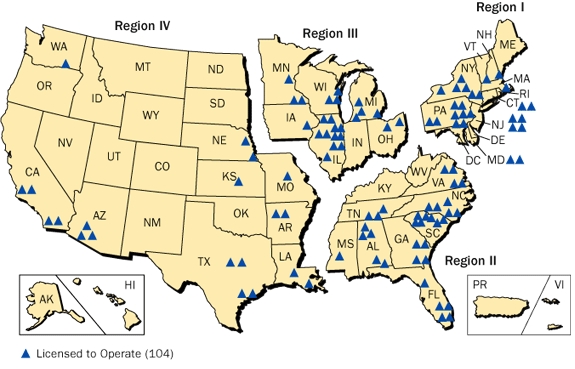
In 2014, nuclear power accounted for
19.47% of the total electrical energy generation of the United States. This 19.47% (or 797 Terawatt-hours) was produced by just 99 commercial reactors. In 2015, there were five new reactors being built. However, even considering those five currently being built, 33 reactors have been permanently shut down since the 1970s. Due to disasters like the
Three Mile Island accident in 1979 or the
Fukushima Accident more recently in 2011 after the earthquake, nuclear power hasn't exactly been a fan favorite for electricity generation.
Nevertheless, nuclear power remains an important power production method. Lifecycle analysis' of nuclear power plants shows that they produce some of the
lowest levels of greenhouse gases when compared to all other power generation methods. So in order to ensure that the benefits of nuclear power generation outweighs the potential dangers, research is being done to find how to lessen the risks associated with nuclear energy.
Michael Tonks, an assistant professor of mechanical and nuclear engineering at Penn State, is exploring new materials to use for nuclear fuel in order to make current light water commercial reactors safer. To put this into a little bit of context, light water reactors use your typical water as coolant. This is in contrast to heavy water reactors, which use deuterium oxide (D
2O, or heavy water) as a coolant.
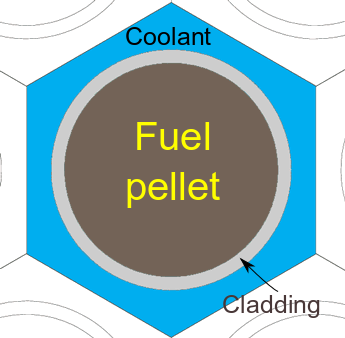
Tonks is looking into altering two different aspects of the fuel: the fuel (pellet) itself as well as the cladding that surrounds the fuel.
The fuel pellet is pretty self explanatory; it is traditionally uranium dioxide (UO
2). The cladding around the pellet is a metal sheath that surrounds the fuel pellet, separating the fuel from the coolant in the reactor. Tonks believes that altering the fuel and the cladding is the "most cost-effective and near-term solution" that could change the course of nuclear energy.
Going back to the fuel pellet; even though uranium dioxide is what is typically used today, it may not be the best option, especially when looking at the safety of the reactors. Uranium dioxide tends to trap heat inside itself (due to its low thermal conductivity), which may lead it to overheat when coolant is lost (which isn't so good, to say the least). Zirconium alloy, which is traditionally used as the cladding, tends to react with water and release hydrogen gas (think big flames at this point).
 |
We don't really want this in our nuclear reactors...
gif courtesy of /r/gifs |
Tonks is trying to find better materials to use for both of these tasks. Utilizing computational models, he is able to model a material's behavior under the conditions it may undergo in a reactor. For example, in the cladding, his team has found a simple solution in layering other materials over the zirconium alloy in order to mix the strengths and weaknesses of the different materials. This layering would prevent the evolution of hydrogen gas in the reactor, thus no possibility for boom. Other alternatives they're looking into include completely scrapping the zirconium cladding and switching to a silicon carbide (a simple compound of one atom of Silicon and one of Carbon) composite.
As for the fuel itself (and the not heating up and melting issues), Tonk's team is trying to find various fuel additives for the uranium dioxide, and utilizing modeling, find out what may happen when the fuel with the additives are exposed to the extreme reactor conditions.
Tonks is using a research structure that many groups are also using: mixing both computational and experimental experiments. Computational research can go through and shrink the pool of possibilities that experimentalists have to try, leading to much more efficient research. While there are many individuals who view one method of research (computational vs. experimental) superior, the fact is that a mixture of both produces the best results.
If you'd like to look deeper into Tonk's research, as well as the research of the people he's collaborating with, you can look at his
website.